Fda Guidance Bcs Classification
Posted : admin On 6/1/2019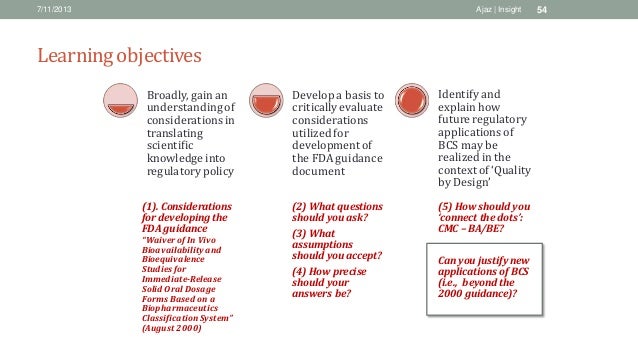
This guidance is developed for immediate release (IR) dosage forms and is. Drugs (BCS class 2), a two-point dissolution specification, one at 15 minutes to. FDA Guidance for. Industry: Dissolution. Specification Setting for BCS Class 1 and 3. Agilent Technologies. Dissolution Systems. Oct 27, 2017 - The FDA guidance on application of the biopharmaceutics classification system (BCS) for waiver of in vivo bioequivalence (BE) studies was.
- Docket Number:
- FDA-2018-D-2614
- Issued by:
The Food and Drug Administration (FDA or Agency) is announcing the availability of a final guidance for industry entitled “Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form Drug Products Containing High Solubility Drug Substances.” This guidance has been developed to provide manufacturers with recommendations for submission of new drug applications (NDAs), investigational new drug applications (INDs), or abbreviated new drug applications (ANDAs), as appropriate, for orally administered immediate-release (IR) drug products that contain highly soluble drug substances. The guidance is intended to describe when a standard release test and criteria may be used in lieu of extensive method development and acceptance criteria-setting exercises.
Bcs Class
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
Bcs Classification Database
If unable to submit comments online, please mail written comments to:
Premium 12-Input 2/2-Bus Mixer with XENYX Mic Preamps & Compressors, British. Plug-ins and ultra-low latency driver downloadable at www.behringer.com. Behringer software download. Aug 7, 2016 - I went onto the website and searched for the correct drivers for this mixer, and saw that there was only the ASIO4ALL driver in the drivers section, and there was no longer an actual driver for the mixer.
Division of Dockets Management (HFA- 305)
Food and Drug Administration
5630 Fishers Lane, Rm. 1061
Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2018-D-2614.
Bcs Class 2 Drugs List
Regulated Product(s)